Andres Cardenas PhD, MPH
- Assistant Professor in Residence, Environmental Health Sciences

https://publichealth.berkeley.edu/people/andres-cardenas/
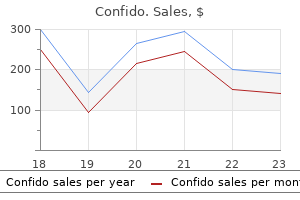
Confido dosages: 60 caps
Confido packs: 1 bottles, 2 bottles, 3 bottles, 4 bottles, 5 bottles, 6 bottles, 7 bottles, 8 bottles, 9 bottles, 10 bottles

Purchase online confido
An instance of this strategy is a current transcriptomic study that was carried out using a panel of 35 inbred strains (Harrill et al. In that research, strains had been recognized as either delicate or resistant to a excessive oral dose of acetaminophen. This evaluation allowed for discernment of transcripts with expression that changed: (i) with remedy, however not with pressure; (ii) with strain, but not with remedy; and (iii) with therapy and strain and that various with the quantity of necrosis. Boxes represent the median and interquartile vary, and whiskers cowl the whole data vary. This similar experi mental paradigm could presumably be extended to metabolomic or pro teomic analyses to determine accessible biomarkers that correlate with toxicity. A recent dem onstration of this software is a study that utilized a panel of inbred strains to examine the molecular mechanisms of genetic sensitivity to isoniazidinduced steatosis (Church et al. In that examine, a mixed transcriptomic, metabolomic, and pharmacoge netic evaluation was utilized to present proof for a novel speculation that isoniazid will increase the capacity for formation of lipid droplets while concurrently decreasing the capability for exporting saved fat from the hepatocytes in sensitive strains. An important aspect of this method is that a subset of sensitive and resistant strains could also be selected for down stream analysis after an preliminary screen of a larger strain panel. In lieu of agency guidelines, general considerations for examine designs based on the previous decade of research investiga tions will be presented. Where mouse information are lacking entirely, it could be beneficial to conduct a dose rangefinding study in a small variety of animals to determine a dose that may restrict undesired antagonistic effects. Because mouse inhabitants research require a massive quantity of animals, con ducting this step on the entrance end could produce cost savings down the highway. As more research are con ducted, estimates of the frequency of those occasions will turn out to be more available. Depending on the study goal, there are a quantity of standards which will come into play upon deciding on a dose for such a research, and no clear guidelines are but obtainable from the regulatory businesses. The potential for a constructive consequence rests, in part, on a speculation that a mouse populationbased strategy will embrace some genetically sensitive people that will manifest the toxicity of concern. There is also the potential for ge netic contamination, spontaneous mutations, and genetic drift to occur even in an established line if the strain has been maintained over many generations (Wiles and Taft, 2010). For these causes, some vendors now "reset" strains after a defined variety of breeding gen erations using cryopreserved embryos derived from the unique inventory. A major consideration for the pattern size is that the magnitude of the effect of a gene var iant is usually not recognized a priori. In distinction, loci that account for <5% of trait variance may require as much as a thousand mice (Gatti et al. This the numbers of animals used for a study is very dependent upon the targets of the research, magnitude and direction of the measured effect, anticipated variation within the impact, desired sig nificance degree, and energy. For the purposes of this chapter, pattern sizes for pharmacoge netic research would be the major focus. Essentially, the algorithm makes use of local phylogenies constructed in genomic areas that exhibit no proof of historic recombination (Pan et al. This is as a result of inclusion of these strains affects the popu lation construction such that many false positives might arise merely due to the genetic divergence of these strains. Because mice of varied genetic contexts will probably exhibit completely different outcomes, it is essential to choose a phenotype for which sampling and measurement error may be minimized. The extra exact the quantitatively measured end result is, the much less noise there will be within the information set and the better the estimate of variance will be. This is as a outcome of the quantitative phenotype info shall be utilized as a beginning enter for the genomewide affiliation models. Special consideration should be given to utilizing bloodbased leakage biomarkers as there may be variations in clearance rates among genetically diverse animals that might affect endpoint measures. This distinction turns into essential as a outcome of it might be necessary to conduct a study during which many biomarkers and outcomes are measured. Here, a few of the analysis packages that have been utilized in recent investigations are introduced. A greater frequency of the variant in the toxicity cases and an increased odds ratio for mutation carriers may signify utility for a pharmacoge netic check. In circumstances where the trait is polygenic, it may be useful to think about a pathwaybased approach.
Buy 60 caps confido fast delivery
A diagnostic toxicogenomics liver injury examine was designed to correlate gene expression adjustments with acet aminopheninduced hepatocellular necrosis throughout 36 strains of mouse (Harrill et al. In this research, 36 inbred strains of mouse have been administered a single 300 mg/ kg dose of acetaminophen, and livers had been subsequently collected for histopathology and toxicogenomic evaluation. Analysis of the toxicogenomic data yielded a panel of 26 genes, which correlated well with liver necrosis in a strainindependent manner. Pathway anal ysis revealed that genes making up the liver necrosis panel have been considerably enriched in pathways related to cell demise and proliferation, together with genes involved in cellcycle regulation. This examine demonstrated that a toxicogenomics approach was able to diagnose acute liver damage regardless of confounding elements corresponding to severity of histopathology findings or genetic background. Another instance of the usage of toxicogenomic information for diagnostic purposes is identification of expression changes correlating with druginduced renal tubular toxicity in rats (Jiang et al. The most appropriate genes for classification may not be informative as regards the organic mechanism. There are many nonoverlapping gene sets, probably due to coregulation, that will present good classification performance for a given endpoint (Natsoulis et al. Due to the intentionally sparse nature of the signatures chosen by many algorithms, biological relevance is mostly not extractable. When multiple nonoverlapping signatures for a given endpoint are analyzed, nevertheless, the combined gene lists do point out that the signatures are derived from biologically related expression modifications (Natsoulis et al. Although histopathology and biochemical endpoints/serum biomarkers exist for diagnosing druginduced renal toxicity, this instance demonstrates the idea of generating and validating a diagnostic toxi cogenomic with potential utility in numerous areas of drug discovery toxicology. Many proponents of diagnostic toxicogenomics imagine that essentially the most priceless use of this approach in drug discovery toxicology is to be able to acquire a biological sample from an easily accessible surrogate tissue. Advantages include the ability to acquire mech anistic gene expression data and to potentially determine particular kinds of goal organ injury. Disadvantages embody the increased labor and value of conducting a toxi cogenomics experiment in comparability with conventional scientific chemistry. In one instance of the utilization of blood as a surrogate tissue for liver, publicly obtainable gene expression information had been mined in order to identify subsets of genes, which were commonly regulated in blood and liver following acetaminophen induced hepatotoxicity. The evaluation revealed a panel of 760 genes at 6 h following acetaminophen administration, and 185 genes at the 24 h time point, which have been regulated in a similar method in each tissues. Moreover, genes within the 6 and 24 h panels have been considerably enriched in ontologies related to the identified mechanism of acet aminopheninduced hepatotoxicity, together with mitochondrial dysfunction and immune response (Zhang et al. In a followon human medical study, sufferers had been administered a four g bolus dose of acetaminophen, and blood was collected for transcriptome evaluation (Fannin et al. While diagnostic toxicogenomic biomarkers show nice promise and utility in these kind of proofofconcept studies, translatability to routine use within the drug discovery testing paradigm or utility to human well being has not but turn into mainstream. The business premise introduced by these endeavors was that large knowledge bases, which combine toxicogenomic knowledge from hundreds of drugs/chemicals with traditional toxicology endpoints (clinical chemistry, hematology, histopathology, molecular pharmacology, etc. If these patterns were generated by utilizing toxicogenomic knowledge, which was collected prior to the onset of a selected poisonous endpoint in a particular tissue, a predic tive signature might be generated. By evaluating a toxi cogenomic pattern generated from an unknown compound towards the signature information patterns within the database, it would then be possible to match towards these predictive signa tures, and primarily based on a optimistic predictive value rating, estimate the chance of a particular toxicity occurring with the unknown compound. Although the toxicogenom ics service enterprise mannequin was difficult to keep, the efforts put forward by these corporations resulted in driving the expertise of predictive toxicogenomics forward to a degree the place this approach was thought of valid and thus became built-in into the drug discovery toxicology take a look at ing paradigm. Case studies that demonstrate the accuracy of predictive toxicogenomic signatures are good proof ofconcept examples of tips on how to greatest generate predictive signatures. One cause for the dearth of use of predictive toxicogenomic signatures is that accuracy of the predictive signature might decrease if the test situations used within the experiment are different from the take a look at situations used in generating the signature. Moreover, whereas many published predictive toxicogenomic signatures were derived utilizing various constructive and adverse class com pounds, there are numerous potential mechanisms driving a particular toxic endpoint, and all mechanisms will not be coated by the signature. Many examples exist however in which a strong set of data was used for derivation of predic tive toxicogenomic signatures and cross and forward validation studies have shown larger predictive sensitivity, specificity, and overall accuracy than different currently accepted methods. Derivation of this signature was accomplished using a large information set consisting of 15 nephrotoxic and 49 nonnephrotoxic com kilos. Rats were dosed every day for both 5 or 28 days, and dose levels were chosen on the basis of the observation of nephrotoxicity determined by histopathological evaluation on day 28 that was not present on day 5. Using a sparse linear programming algorithm, the authors generated a 38gene signature.
Purchase confido once a day
These are time and useful resource intensive and customarily low throughput assays ensuing in their implementation later within the development process, when more resources are released to study the few molecules which have superior to this stage. Now, early within the discovery part, utilizing human enzymes and humanorigin cells, drug discovery programs are able to get hold of extremely actionable information about the druglikeness of new molecules, the potential to attain goal organ, and early indications of known human mechanisms of toxicities. Bioavailability, tissue distribution, pharmacoki netics, metabolism, and toxicity are assessed typically in a single rodent and one nonrodent species previous to administering a drug to a human to consider drug pharmacokinetics and exposure in a medical trial (phase 1). This division is also a perform of costeffectiveness and the need for the specific data. It is useful to begin from the final word aim, which is often a therapeutic drug for a particular affected person inhabitants and from there work backwards towards discovery. If a compound has excessive target receptor binding and biological activity in cells and in relevant in vivo animal models, what are the chances of it changing into a successful drug In order to attain it, a molecule have to be in solution, and thus step one is often to assess the sol ubility of a compound. Chemical and metabolic sta bility is an additional extension of the intrinsic properties of a molecule. Chemical stability in buffers, simulated gastric and intestinal fluids, and metabolic stability in plasma, hepa tocytes, or liver microsomes of different species could be mea sured to predict the rate of breakdown of a compound in the totally different environments encountered within the human body on the way to its target. Measurement of permeability throughout a cell monolayer corresponding to Caco2 mannequin is a good predictor of excessive human oral bioavailability. This is crucial as only unbound medication are capable of attain the target and exert their fascinating pharmacologic and undesirable (toxic) results. However, because of enzyme inhibition, cytotoxicity, or other effects of some compounds, negative results generated from measuring enzyme exercise could be false unfavorable. Highquality plateable cryopreserved human hepatocytes should be used to generate results, that are acceptable for regulatory filing. The regulatory businesses also beneficial that the assay should use hepatocyte prepara tions from at least three donors. As a outcome, metabolism could be exploited to produce a better drug, which is able to influence the medicinal chemistry strategy. The identification of drugmetabolizing enzymes involved within the main metabolic pathways of a compound assists to predict the possible drug�drug interactions in people. This data additionally could additionally be used to design human clinical trials to detect unneces sary drug�drug interplay. Another household of molecules that influence drug exposure and subsequently potential opposed occasions are drug transporters. Over the past 25 years, a quantity of important human drug transporters have been recognized which might be expressed at the apical or basal aspect of the epithelial cells in numerous tissues (Ambudkar et al. Major human transporters and examples of medicine reported to be substrates, inhibitors, or inducers of those transporters are listed in Table 7. The impact of drug transporters on permeability and the effect of medicine on trans porter exercise can be measured in cells which have been dem onstrated to categorical such transporters endogenously. Early knowledge about these interactions is instrumental to the medicinal chemistry technique and helps drive lead optimization. Among the remaining (extensive metabolizers), enzyme exercise is extremely variable, from extraordinarily excessive in ultrarapid metabolizers to markedly reduced in intermediate metabolizers. Many chal lenges stay in our understanding of the medical relevance of genetic variations in transporters to drug disposition and drug�drug interactions. Greater than 50% of this attrition resulted from failure to reveal efficacy in section 2 studies. Compounds with demonstrated efficacy in opposition to a target in vitro and in animal fashions frequently proved to be ineffective in humans. Ideally, this information would be obtained as early as potential to focus resources on compounds more than likely to reach the target organ. Unfortunately these strategies have limitations, including requiring subtle tools, technical expertise, mathematical modeling, species differences, invasiveness, and low throughput-which render them unsuitable for use throughout early stages of drug discovery. This new mannequin consists of major cultures of human mind capillary endothelial cells cocultured with major human glial cells (Megard et al. One important step within the improvement of any in vitro model is to correlate in vitro and in vivo data in order to val idate experimental models and to assess the predictive energy of the strategies (Pardridge et al.
Order confido online pills
For novel biomarkers this is usually achieved by including an exploratory goal that examines the feasibility of measuring the biomarkers in the examine inhabitants. Within the protocol a summary of preclinical or translational proof for the biomarkers must be provided. When available, biomarker qualifications or letters of assist from regulatory authorities should be referenced. A schedule of occasions describing when samples for biomarker analysis must be collected in context with all other medical visits and tests should be included. When special collection, processing, or storage issues are required, a separate Drug Discovery Toxicology: From Target Assessment to Translational Biomarkers, First Edition. The study sponsor assembles a team to combine pattern collections at the level of clinical care with biomarker storage and evaluation utilizing validated assays. The samples may be submitted for instant biomarker evaluation and/or could also be biobanked for future analysis. Data generated from biomarker evaluation is collected and saved and forwarded to the information group for evaluation utilizing the statistical approaches described within the study sponsor protocol. The combination of traditional and novel preclinical security (pharmacokinetic and/or pharmacodynamic) biomarkers is frequently utilized in phase I research, utilizing a translatable evidencebased strategy. Moreover, sponsors may demonstrate the benefits from fitforpurpose biomarkers when rigorously evaluated in accordance to regulatory guidance Table 42. When translating a biomarker from preclinical to clinical studies, the species specificity of the assay must be evaluated. Differences in amino acid sequence homology or posttranslational modifications between animal species and human proteins might end result within the want for use of a speciesspecific assay. Establishing the popular matrix for biomarker analysis is paramount (see Chapter 40). Regardless of species, assortment of whole blood for isolation of plasma and serum is semiinvasive and infrequently related to restricted collection volumes (Diehl et al. Collection of urine is minimally invasive and permits for collection of large pattern volumes. The kinetics of security biomarkers has not been extensively studied; due to this fact, frequent sampling may be informative. However, sampling frequency in the scientific setting ought to be balanced with concerns for varied factors together with but not limited to affected person consolation and ease of assortment. Understanding and controlling components that ensure correct and constant biomarker outcomes often helps prioritization of biomarkers to be included in a research and choice of research site. For instance, hydration standing, medicine, food plan, tobacco use, exercise, concomitant diseases, or comorbidities may confound interpretation of biomarker values. Capturing this sort of data at the time of pattern collections may help in identification of patient related confounding elements that influence biomarker values and interpretation. The operations team also wants to resolve whether extra biofluid sample volumes should be collected and banked for future retesting (as appropriate) and for bridging or crossassay validation studies. If future exploratory research are also in scope, the quantity of tissue biopsy sample and variety of biofluid aliquots per sample type should be predetermined for longterm biobanking. For drug security biomarker assessment, every effort must be taken to minimize danger to the affected person together with identification of the least invasive method for tissue biopsies. The choice of needle measurement impacts threat of bleeding, discomfort, and ache for the affected person, and the quantity of tissue material obtained for biomarker evaluation is organ dependent. The 16G needle was found to provide one of the best balance between usefulness and patient consolation. For a evaluate of points associated to liver biopsy methods, the reader is referred to Strassburg and Manns (2006), Friedman (2004), and Copel et al. For skeletal muscle biopsies, the percutaneous biopsy approach has been reported to be safe and supply enough tissue for evaluation (Shanely et al. For some patients, venipuncture could also be difficult, especially if frequent collection time points are needed. However, guidance for flushing the catheter port should be offered as this may result in dilution of the sample or end in contamination with anticoagulant. A number of blood assortment units are available and should have an result on the biomarker evaluation as mentioned beforehand (see Chapter 40) and reviewed by Bowen and Remaley (2014).
Safe 60 caps confido
If ache medications-particularly narcotic-class medications- are used for a protracted period, the dysphagia clinician should additionally contemplate gastroparesis in the profile of potential swallowing deficits. In addition, clinicians should be conscious that ache inside the swallowing mechanism may end up from fungal infections or peripheral nerve harm. As pain diminishes with appropriate medical treatment, patients with decreased oral consumption attributable to odynophagia ought to improve each the quantity and variety of meals and liquids taken by mouth. Xerostomia also has a direct and negative impact on mastication of more stable foods. The capacity to fee the severity of xerostomia provides an goal dimension to the clinical analysis of the affected person with head and neck most cancers. Clinicians must also focus on with the affected person his or her notion of the impact of xerostomia on swallowing and different oral capabilities. Taste is mediated by the tongue, with only five basic tastes recognized (sweet, sour, bitter, salty, and umami). Altered senses of taste and odor can have a direct, unfavorable influence on oral intake because patients will keep away from foods which may be perceived as aversive. Although normal protocols exist for the systematic analysis of taste and scent function, affected person report is often enough to doc the presence of those sensory deficits and their results on oral intake of food and liquid. If impaired senses of style or scent are determined to be a main factor affecting decreased oral consumption, patients ought to be referred to an applicable oral health professional for extra in depth analysis and potential remedy. Soft tissue of the skin and muscle can turn into fibrosed, which reduces motion within the swallowing mechanism and within the head and neck region normally. Dysphagia clinicians ought to attempt to differentiate the underlying reason for lowered motion primarily between muscle weak spot and tissue fibrosis. The affected person with soft tissue fibrosis demonstrates onerous, or "woody," presentation of a region that has been irradiated such because the anterior facet of the neck. Simply grasping both sides of the larynx and making an attempt to transfer this construction from facet to aspect offers some indication of the degree of motion and hence fibrosis. Subsequently, the clinician can try to really feel laryngeal motion during a volitional swallow. The mixture of reduced passive and volitional movement means that fibrosis may be a limiting issue. If attainable, endoscopic inspection of the larynx and pharynx helps decide whether the effects of fibrosis are restricted to the superficial skin and muscle tissue or if deeper buildings are involved. More particulars of this evaluation are supplied in Chapter 8, but the key feature is to evaluate motion within the larynx, pharyngeal partitions, and the base of the tongue. Strictures on this phase cut back the sphincter opening and limit the quantity of meals the patient is ready to swallow. This impairment ought to be thought of in sufferers with head and neck most cancers who report problem swallowing strong foods. Finally, a common and potentially debilitating type of fibrosis can lead to decreased mouth opening, or trismus. Trismus may result from decreased flexibility of the masseter and temporalis muscle tissue, which are the first muscular tissues of jaw closure. If these muscle tissue turn out to be fibrotic, they pose a substantial force in opposition to the muscle tissue of jaw opening and restrict the diploma of vertical opening of the mouth. This scenario can negatively affect mastication, swallowing, speech, and common oral care. As previously talked about, a vertical mouth opening of lower than 35 mm may be considered lowered and indicative of trismus. One scientific tool that could be used to assess the effect of trismus on activities of daily living is the Gothenburg Trismus Questionnaire. Reasons for malnutrition may embrace dysphagia, odynophagia, style deviations, poor urge for food (which in itself may be multifactorial), elevated caloric wants, or other metabolic, bodily, or psychological components. Weight change is a common guideline for dietary change, and unintentional weight reduction is usually used as a clinical sign of potential dietary threat.
Green Ozier (American Dogwood). Confido.
- Dosing considerations for American Dogwood.
- How does American Dogwood work?
- Are there safety concerns?
- What is American Dogwood?
- Headaches, fatigue, weakness, fever, chronic diarrhea, loss of appetite, malaria, treating boils and wounds, and other conditions.
Source: http://www.rxlist.com/script/main/art.asp?articlekey=96525
Purchase confido 60 caps with visa
Smallmolecule metabolites are represented by purple hexagons, and reactions are represented by grey rectangles. Gene/protein objects are represented by other various shapes and colours relying on their functional annotations. Green arrows represent activation, pink arrows represent inhibition, and grey arrows characterize different kinds of unspecified interac tions. Thirdly, the suitability of the organ or cell kind used to monitor the toxicological response ought to be taken into account. For example, whereas an in vitro cell system could additionally be useful to derive signatures for nuclear receptor activation. Similarly, gene expression profiling in the liver is unlikely to provide robust classification of particular cardiotoxic outcomes. In some cases, nonetheless, crossorgan signatures of toxicity may be possible, and certainly desirable, for instance, within the prediction of druginduced liver harm in humans based mostly on expression profiling in blood (Fannin et al. Such analyses should be undertaken with great care to the experimental design and cautious validation of the resulting signatures. Finally, as beforehand talked about, some endpoints are more addressable by the signature approach than others, and a full understanding of the realworld performance charac teristics of any given signature by way of its ability to cor rectly determine each toxicants (sensitivity) and nontoxicants (specificity) is necessary to have the ability to appropriately interpret the classification predictions generated on new information with novel compounds and coverings. Ideally this is accom plished with ahead validation of the signature utilizing a 45. When properly designed, executed, and validated, the use of genomic signatures can present a rapid, sensitive, and highly effective method to interpret toxicogenomic data and provide predictions on toxicological outcomes and mecha nisms of action. Gene signatures can help within the interpretation of complicated gene expression knowledge by deconvoluting them into discrete outcomes that are more readily comprehensible to pharmacologists and toxicologists nonexpert in gene expres sion analysis. Early adopters of this expertise were usually overly optimistic about the potential applications of this strategy, although little was identified about the applicability or even what types of toxicological questions might realistically be answered. Due to excessive cost, issue in conducting evaluation of large quantities of data, and inappropriate use of the expertise, the sphere of toxicogenomics has usually been accused of not living up to the original promise of what type of knowledge may doubtlessly be delivered (Chen et al. Although many obstacles to the routine use of toxicogenomics in drug discovery toxicology still exist, retrospective analyses of case studies present that even with these obstacles, use of the technology has made a optimistic impact on reducing attrition because of questions of safety and contrib uting to mechanistic understanding of observed toxicities in exploratory toxicology research (Foster et al. A evaluation of case research in the usage of toxicogenomics in drug discovery reveals three major categories of application: diagnostic toxicogenomics (biomarkers), predictive toxi cogenomics, and mechanistic/investigative studies (Searfoss et al. In this part, case research examples of each of those use classes might be mentioned. This method has the best utility when other primary biomarkers of toxicity. In utilizing toxicogenomics in a diagnostic method, a number of forms of knowledge analysis may be used, including induction/repression of particular person genes of interest, evaluation of specific toxicity pathways or de novo networks, or use of gene signatures. Predictive toxicogenomics has higher potential value than diagnostic toxicogenomics. In the early days of toxi cogenomics, nonetheless, predictive toxicogenomics was one of the most overhyped and least correct uses of the tech nology. The premise underlying predictive toxicogenomics is that gene expression changes, which happen prior to the onset of a pathology endpoint, can be used to predict what endpoints shall be modulated at some time after collection of the tissue (Foster et al. A second approach to predic tive toxicogenomics is using gene expression in one mannequin or species to predict what toxicities will occur in an alternative model-for example, monitoring gene expres sion in main hepatocytes to predict what toxicities will happen in the liver throughout a repeatdose in vivo toxicology examine or using rat toxicogenomic knowledge to predict whether or not an identical finding will occur in a human medical trial. In predictive or prognostic toxicogenomics, the information analysis methodology most often employed is use of multigene, informati cally derived gene signatures. An further example of the use of predictive toxicogenomics is when data is generated in cells or tissue with one compound, and these data are used to predict the forms of toxicity that can occur with a similar molecule or a compound from a special chemical series. The third example of the use of toxicogenomic technol ogies in drug discovery is in investigative toxicology studies where mechanisms of toxicity are characterised. With continual advances in pathway evaluation and web work generation in silico/computational tools for data anal ysis, understanding mechanisms of toxicity has become extra routine and accessible to toxicologists with restricted experience in bioinformatics. Moreover, mechanistic toxicogenomics provides the opportunity for drug discovery toxicologists to characterize mechanisms of toxicity associ ated with a particular molecule and then use the data to inform medicinal chemists which particular off targets to keep away from, ensuring that the following generation of backup molecules in a program have a lowered threat of a particular toxic endpoint. The use of toxicogenomics as a diagnostic tool is usually not warranted, as the gold normal scientific chemistry, hematology, and histopathology endpoints are generally adequate for diag nosing a particular toxicity. There are, nonetheless, circumstances during which a diagnostic toxicogenomics strategy has provided priceless information to complement the frequent endpoints. Splitsample crossvalidation evaluation revealed the signature to have predictive sensitivity and specificity of 83 and 94%, respectively.
Buy confido 60caps visa
The obvious lack of the need for exogenously added Wnt ligands means that the mix of shear stress and cyclic mechanical pressure could induce Wnt signaling pathways. Further investigation into the process of differentiation in this system will provide a lot needed information to underneath stand the mechanisms of differentiation induced by shear stress and mechanical strain and be helpful to evaluate with the differentiation processes identified to date for 3D organ oid constructions. Despite the limitations of each cell lines and first cells, they are often useful as easy models and have the added advantage of being excessive throughput. Early goal assessment or hypotheses from in vivo findings can aid in understanding of the need for simple or advanced models in addition to assist within the number of an applicable cell line for in vitro mechanistic and screening toxicology studies. Common questions we ask when defining the appropriateness of an in vitro model are: Is the target expressed within the cell line Are we derisking a lesion that seems to be regionally specific and thus has the potential want for celltype specificity and first cell isolation Or is the hypothesized mechanism sufficiently basic that we may use a nonspecific and easy cell model These assays are particularly fitted to enterocytebased toxicity mechanisms and can be utilized to any major cell or line. They have the drawback of not providing much mechanistic data however is normally a helpful preliminary screen for giant sets of compounds significantly if they can be combined with data sets containing completely different chemical sequence and totally different goal actions to determine whether the toxicity is on or off target. Molecularly, the epithelial barrier is maintained by tight and adherens junctions and desmosomes between enterocytes (reviewed in Peterson and Artis, 2014). Cell migration could be measured in vitro with a scratch wound assay, transmem brane/Boyden chamber assay, cell exclusion assay, or micro fluidic chamber assays (Hulkower and Herber, 2011). In a scratch wound assay, a cell monolayer is "wounded" or scratched, and the motion of cells into the cleared area is monitored over time. Cell migration from the apical to basal chamber of a transwell system is measured in a Boyden chamber migration assay usually with the application of a progress stimulus within the basal chamber as the stimulus to migrate. Cell exclusion assays are much like scratch wound assay in that the movement of cells into a cleared space is measured; the difference is that instead of wounding the monolayer, a physical barrier is positioned into the well previous to plating the cells, and after software of the suitable stimulus, the barrier is eliminated, revealing a transparent space into which cells can migrate (Hulkower and Herber, 2011). The problem deepens after we try and translate in vitro assay findings throughout a number of species. Systemic treatmentinduced gastrointestinal toxicity: incidence, medical presentation and administration. Modulation of dendritic cell phenotype and function in an in vitro mannequin of the intestinal epithelium. Functional cell models of the intestine and their applications in food microbiology-a evaluate. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in people. The mammalian intestinal epithelium as integral player in the establishment and mainte nance of hostmicrobial homeostasis. Risks of serious infection or lymphoma with antitumor necrosis issue remedy for oediatric inflammatory bowel dis ease: a systematic evaluation. On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic "bodyonachip" gadgets. The development of a method for the preparation of rat intestinal epithelial cell major cultures. Primary cultures for research of cell regulation and physiology in intestinal epithe lium. Smooth muscle actin as a novel serologic marker of severe intestinal injury in rat intestinal ischemia�reperfusion and human necrotising enterocolitis. Comparative anatomy, physiology, and mech anisms of illness production within the esophagus, stomach, and small gut. Wnt5a signaling promotes apical and basolateral polarization of single epithelial cells. Target organ toxicities in studies performed to support first time in man dosing: an evaluation throughout species and therapy areas. Concise evaluate: the relevance of human stemcell derived organoid models for epithelial translational drugs. Druginduced oesophageal issues: patho genesis, incidence, prevention, and management.
Order generic confido line
Large Nested Carcinoma: Necrosis Large Nested Carcinoma: Architectural Distribution (Left) Areas of central necrosis may be seen in giant nested carcinoma, a feature that ought to immediate consideration of malignancy. The dimension and the irregular distribution of the urothelial nests also recommend the potential for large nested carcinoma. Large Nested Carcinoma: Focal Stromal Response Large Nested Carcinoma: Deep Invasion (Left) In uncommon foci, large nested carcinoma could also be associated with a delicate degree of stromal reaction, characterized by a rim of elongated myofibroblasts, as seen in this instance. This high-power photomicrograph demonstrates the comparatively bland cytologic features of large nested carcinoma. The distribution within the lamina propria is more irregularly dispersed than in benign mimics. The reactive stromal modifications and the irregular, randomly distributed nests are features that ought to recommend a diagnosis of carcinoma. Urothelial Carcinoma With Small Tubules Microcystic Carcinoma (Left) this deeply invasive carcinoma has a deceptively bland look with small luminal buildings that mimic blood vessels on low-power analysis. The irregular and haphazard nature of the proliferation, as well as widespread involvement of lamina propria, aid in recognition as carcinoma. Microcystic Carcinoma Microcystic Carcinoma (Left) Microcystic urothelial carcinoma is proven with innocuous nuclear features. The variably sized and formed cysts are lined by flattened to columnar epithelium or multilayered urothelium. In contrast to the everyday pseudostratified columnar lining cells of adenocarcinoma, these neoplastic cells are flattened. Microcystic Carcinoma 390 Overview of Invasive Carcinoma Subtypes Urinary Bladder Plasmacytoid Carcinoma Plasmacytoid Carcinoma (Left) Plasmacytoid urothelial carcinoma is characterised by particular person round neoplastic cells with eccentric nuclei and deep eosinophilic cytoplasm. The neoplastic cells are easily confused with plasma cells or histiocytes at this magnification. Plasmacytoid Carcinoma Plasmacytoid Carcinoma (Left) the massive measurement of the neoplastic plasmacytoid carcinoma cells can be compared to the smaller adjoining regular plasma cells. This pattern may be very troublesome to acknowledge on intraoperative frozen section analysis of margins. Plasmacytoid Carcinoma Cytokeratin: Plasmacytoid Carcinoma (Left) the round eccentric nuclei and the eosinophilic cytoplasm are attribute of plasmacytoid urothelial carcinoma. In difficult circumstances, immunohistochemistry may help in the distinction from inflammatory cells. In this example, the carcinoma involves the wall of the seminal vesicle where it could presumably be mistaken for inflammatory cells. The plasmacytoid variant of urothelial carcinoma has an uncommon, but characteristic, pattern of spread to peritoneal surfaces. Plasmacytoid Carcinoma in Fallopian Tube Plasmacytoid Carcinoma in Periureteral Tissue (Left) When involving gentle tissues. Plasmacytoid Carcinoma in Lymph Node Plasmacytoid Carcinoma in Lymph Node (Left) Metastatic plasmacytoid urothelial carcinoma in a lymph node may be deceptively bland, where it closely mimics sinus histiocytes or intranodal plasma cells. It is very helpful to know that a plasmacytoid morphology is current when screening excised lymph nodes. Cytokeratin: Plasmacytoid Carcinoma 392 Overview of Invasive Carcinoma Subtypes Urinary Bladder Noninvasive Papillary Urothelial Carcinoma Noninvasive Papillary Urothelial Carcinoma (Left) Noninvasive papillary urothelial carcinomas may have a micropapillary structure characterized by thin elongated micropapillae which have a greater length than width. Micropapillary Carcinoma Micropapillary Carcinoma (Left) Invasive micropapillary carcinoma is characterized by back-to-back lacunar spaces containing small nests of carcinoma, as seen in this example. The cytology of the neoplastic cells may be quite bland, despite the deeply invasive progress and the common presentation with metastases. Micropapillary Carcinoma Micropapillary Carcinoma (Left) Micropapillary carcinoma commonly presents at high stage. The tumor maintains characteristic features similar to back-to-back lacunar areas, multiple nests in a single retraction house, and ring varieties. Micropapillary carcinoma of the urinary bladder maintains a urothelial phenotype and sometimes lacks a real fibrovascular core. The back-to-back lacunar spaces and the presence of a quantity of nests in a single lacunar space are other characteristic options. This example exhibits more nuclear pleomorphism than usually seen in micropapillary carcinoma.
References
- Makhlouf GM, Schubert ML: Gastric somatostatin: A paracrine regulator of acid secretion. Metabolism 39:138, 1990.
- Akowuah EF, Davies W, Oliver S, et al. Prosthetic valve endocarditis: early and late outcome following medical or surgical treatment. Heart 2003;89:269-272.
- Maegdefessel L, Spin JM, Azuma J, et al: New options with dabigatran etexilate in anticoagulant therapy, Vasc Health Risk Manag 6:339-349, 2010.
- Lang CC, Beniaminovitz A, Edwards N, et al. Morbidity and mortality in diabetic patients following cardiac transplantation. J Heart Lung Transplant 2003;22:244-249.
- Tatarishvili J, Oki K, Monni E, et al. Human induced pluripotent stem cells improve recovery in stroke-injured aged rats. Restor Neurol Neurosci 2014;32:547-58.
- Park J, Banno S, Sugiura Y, et al. Microscopic polyangiitis associated with diffuse panbronchiolitis. Intern Med 2004;43(4):331-5.